Summary of A New Way To Prevent Common Causes of Vision Loss:
Researchers at UVA Health have discovered a new factor contributing to the harmful growth of blood vessels in the eye, paving the way for new treatments for macular degeneration and other common causes of vision loss. By targeting the epigenetic machinery with the fat mass and obesity-associated (FTO) protein, a key protein that determines VEGF levels, the scientists were able to reduce VEGF levels in lab mice and gain a deeper understanding of how ocular immune cells can cause a loss of control over blood vessel growth under the retina. This breakthrough could lead to the development of cost-efficient. These effective and accessible interventions avoid issues such as drug resistance, which can occur with conventional anti-VEGF therapies used in clinical treatments.
*****
New Study Identifies a Key Protein That Causes Harmful Blood Vessel Growth in Eye
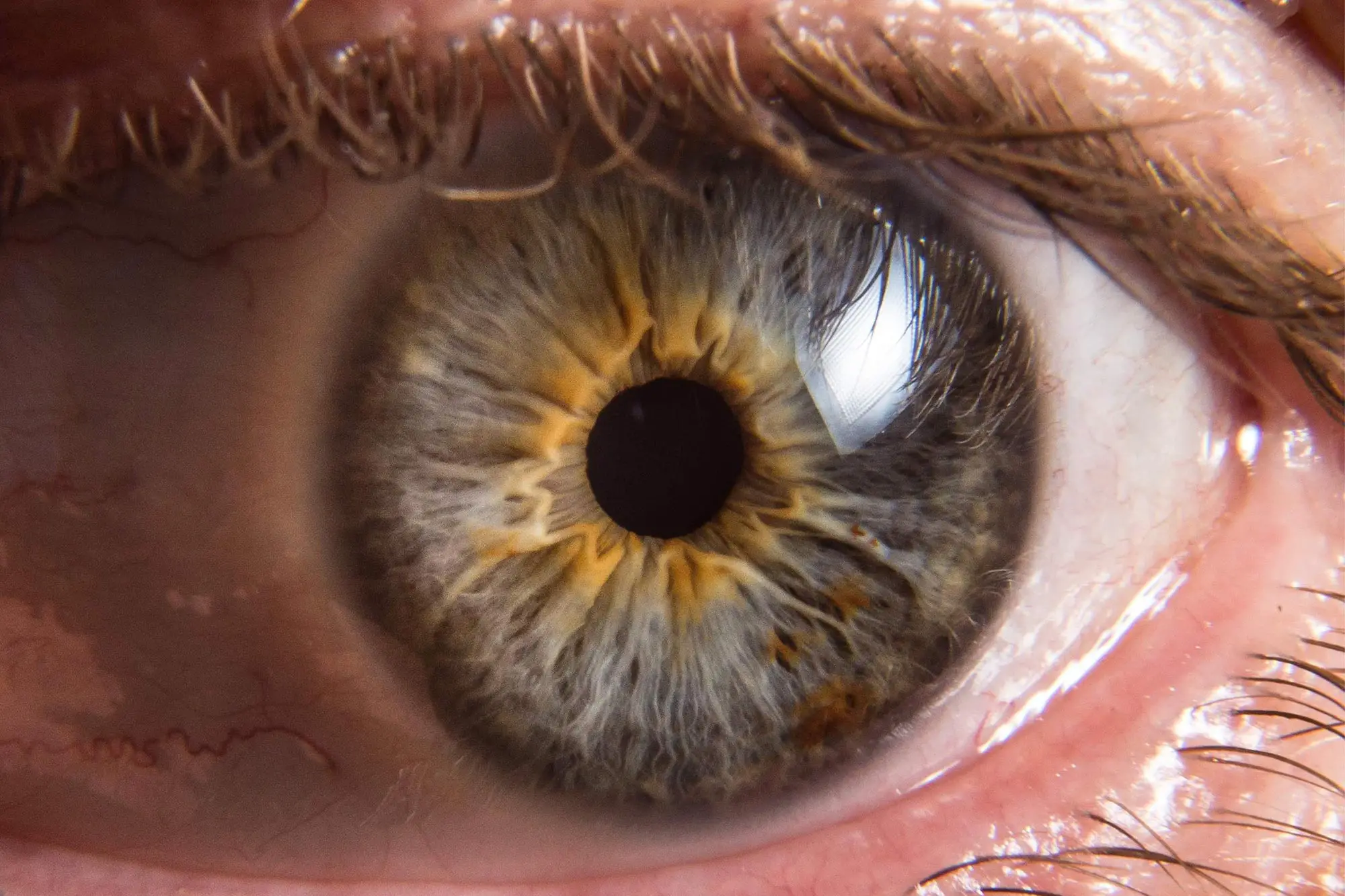
For people with vision loss, abnormal blood vessel growth in the eye, also known as neovascularization, is a serious concern. This growth can lead to vision loss and even blindness if not treated promptly. Scientists at UVA Health have uncovered a previously unknown factor contributing to the harmful growth of blood vessels in the eye. This breakthrough could pave the way for new treatments for macular degeneration and other common causes of vision loss.
Understanding Vision Loss
Scientists have known that abnormal vessel overgrowth in the eye is fueled by excessive amounts of a substance called “vascular endothelial growth factor-A,” or VEGF, which plays an important role in blood vessel formation. Now, treatments that target VEGF to prevent vessel overgrowth are available, and they often provide dramatic benefits at first. Unfortunately, these benefits can fade with time. That leaves doctors needing better treatments to help preserve patients’ eyesight.
A New Target for Treatment
Jayakrishna Ambati, MD, and Shao-bin Wang, Ph.D. of UVA, along with their colleagues, have pinpointed a new target to halt the development of abnormal blood vessel tangles associated with eye conditions like neovascular age-related macular degeneration, proliferative diabetic retinopathy, and ischemic retinal vein occlusion.
“This fat mass and obesity-associated (FTO) protein was previously shown to be correlated with obesity in humans. Unexpectedly, we found it also plays important roles in regulating ocular neovascularization through an epigenetic mechanism,” Ambati said. “This exciting discovery finally answers a longstanding question about how ocular immune cells, such as macrophages, contribute to abnormal blood vessel growth under the retina. Our team first investigated this question 20 years ago, and we’re thrilled to have found an answer.”
The scientists noted that blocking this protein in lab mice reduced their VEGF levels significantly, and it did so in a targeted way, without unwanted side effects.
A Promising Discovery
In addition to identifying a promising target for developing new treatments for vision loss, the discovery sheds important light on the fundamental mechanisms responsible for the blood vessel overgrowth that robs millions of people of sight. Neurovascular age-related macular degeneration alone affects more than 200 million people worldwide. While much more research and testing will be needed before the new finding can be translated into treatment, the UVA scientists are excited about the potential of the discovery.
“Current strategies for treating ocular neovascular disorders, which primarily focus on regulating the protein levels of VEGF, are not perfect. Therefore, it is imperative to identify more targetable candidates to develop alternative therapies,” Wang said. “We hope our study will pave the way for developing new treatments, ultimately reducing the burden of neovascular-related illnesses.”
The study was funded by the National Institutes of Health, the UVA Strategic Investment Fund, the DuPont Guerry III professorship, a gift from Mr. and Mrs. Eli W. Tullis, the Annette Lightner Fund, a BrightFocus Foundation Award, and the Owens Family Foundation.
![Masterclass: Discover Purpose & Achieve Dream Life (Women’s History Month) [EP 1412]](https://defuckmedia.com/wp-content/uploads/2023/03/DSC01644-scaled-150x150.jpg)
Comments are closed