Summary of New Approach Allows Researchers To Hit “Undruggable” Cancer Target:
Researchers at the University of Michigan have found a way to eliminate the “undruggable” protein STAT5, a protein involved in leukemia and other forms of cancer, by utilizing a cellular garbage disposal function. They have identified a protein degrader, AK-2292, that targets and removes STAT5, laying the foundation for its potential as a cancer treatment. The compound effectively stops cell growth in cell lines of human chronic myeloid leukemia (CML) and induces tumor regression in mouse models of CML. The researchers hope to optimize the compound further to advance it into clinical development for treating cancers in that STAT5 plays a major role.
*****
Protein Degrader Demonstrates Success in Targeting “Undruggable” Protein, STAT5
Researchers have sought to target the protein STAT5 in the battle against cancer for decades. Though organizations have spent an insurmountable amount of time and resources on this pursuit, STAT5 eventually became “undruggable.” The good news is that researchers from the University of Michigan Rogel Cancer Center have developed a unique approach to tackling the issue.
STAT5’s Role in Cancer
STAT5 plays a pivotal role in developing and progressing certain blood cancers. But efforts to identify a small molecule inhibitor to obstruct STAT5 have been botched for various reasons. One primary issue is designing a drug that can bind with STAT5 with a high affinity, measuring how well they fit together. When a compound does indeed bind, it may not make its way into the cells and tissue. Finding a combination that inhibits STAT5 exclusively without impacting other STAT proteins is also difficult.
The Search for a Novel Approach
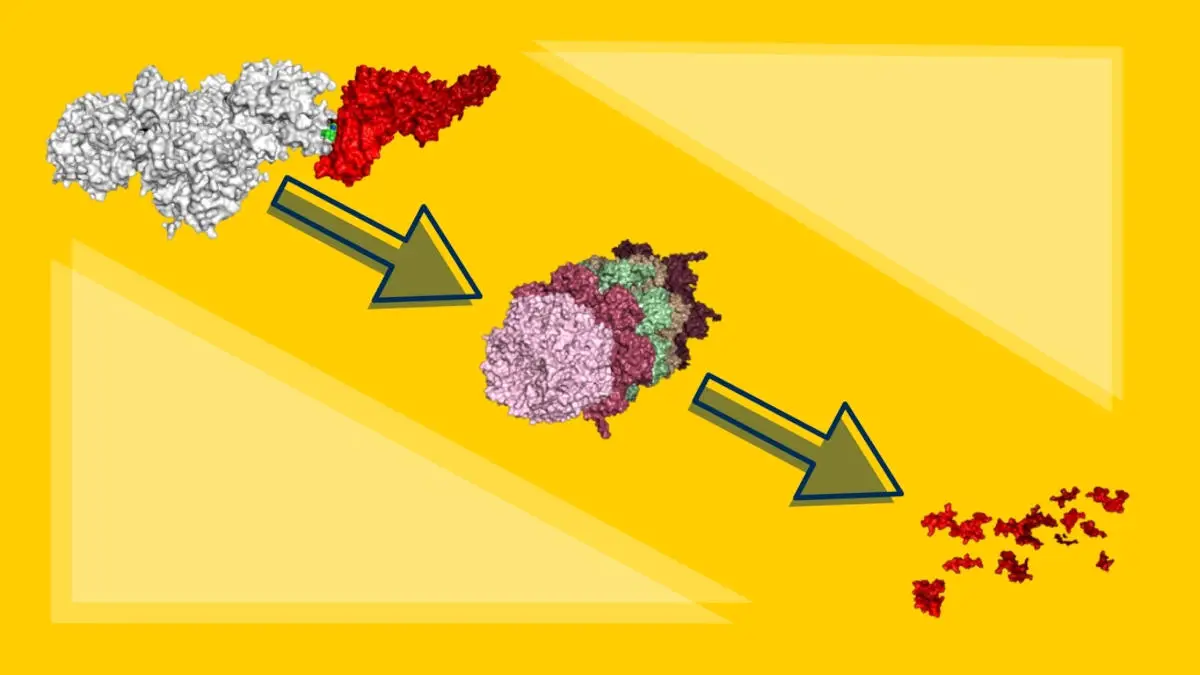
Shaomeng Wang, the Warner-Lambert/Parke-Davis Professor in Medicine and professor of medicine, pharmacology, and medicinal chemistry at the University of Michigan, had an alternate approach. His lab has spent numerous years working on a new drug development approach to target protein degradation. This mechanism is a naturally occurring function inside cells that eliminates unwanted proteins from the body, much like a garbage disposal.
AK-2292 – Protein Degrader for STAT5
Using this approach, Wang’s lab identified a protein degrader, AK-2292, that targets and removes STAT5. The compound was highly specific to STAT5 without affecting other STAT proteins. It effectively eliminated STAT5 proteins from tumor cells and tissues, unlike traditional small molecule inhibitors that bind with the protein and interfere with its function. AK-2292 was effectively taken up by both cell lines and mouse models and effectively stopped cell growth in human chronic myeloid leukemia cell lines and caused tumor regression in mouse models of CML.
A New Direction
“We’ve overcome some of the major issues that were barriers for scientists to target STAT5,” Wang said. “People have worked in this field for the last 20 years, and no small molecules target STAT5 going into clinical development. This study shows us STAT5 can be targeted through a protein degradation approach. It’s a new, exciting direction for developing a potential drug molecule targeting STAT5 for treating cancers in which this protein plays a major role.
“This compound gives us a very solid foundation to do further optimization to identify a compound that we eventually can advance into clinical development,” Wang added.
A Bright Future
Wang’s lab has been investigating protein degraders for several years. It has a plethora of degraders in advanced preclinical development studies, which he hopes will eventually lead to clinical trials for cancer treatment in people.
The University of Michigan has filed patent applications on inhibitors and degraders described in this study, licensed to Proteovant Therapeutics. S.W., A.K., L.B. M.W., D.M., and J.S. are co-inventors on one or more of these patent applications and receive royalties from the University of Michigan. The study was funded by Proteovant Therapeutics, Oncopia Therapeutics (acquired by Proteovant), the National Cancer Institute, the U.S. Department of Energy, and a Michigan Economic Development Corporation and Michigan Technology Tri-Corridor grant.
This work was also supported by these Rogel Cancer Center Shared Resources: Pharmacokinetics, Proteomics, and Structure and Drug Screening.

Comments are closed