Summary of Scientists Shed Light on How Immune Cells Respond to Cancer Cells:
Researchers at the UCLA Jonsson Comprehensive Cancer Center have identified and analyzed how immune cells react to cancer cells, which could lead to improved and personalized immunotherapies. The study used gene-editing technology to observe immune responses in patients with metastatic melanoma receiving anti-PD-1 “checkpoint inhibitor” immunotherapy. The researchers found that immunotherapy directed a diverse repertoire of T cells against a small group of selected mutations in a tumor, with T-cell responses expanding and evolving during treatment. Even in patients who did not respond to therapy, a tumor-reactive T-cell response was induced, potentially offering a source for genetic modification to combat tumors. The researchers believe their findings will help design better treatments and create more successful treatments for those resistant to current therapies.
*****
New Study Could Lead to More Effective Immunotherapies
A recent study by researchers at the UCLA Jonsson Comprehensive Cancer Center has made promising strides in understanding how immune cells interact with cancer cells, potentially leading to more personalized and effective immunotherapies.
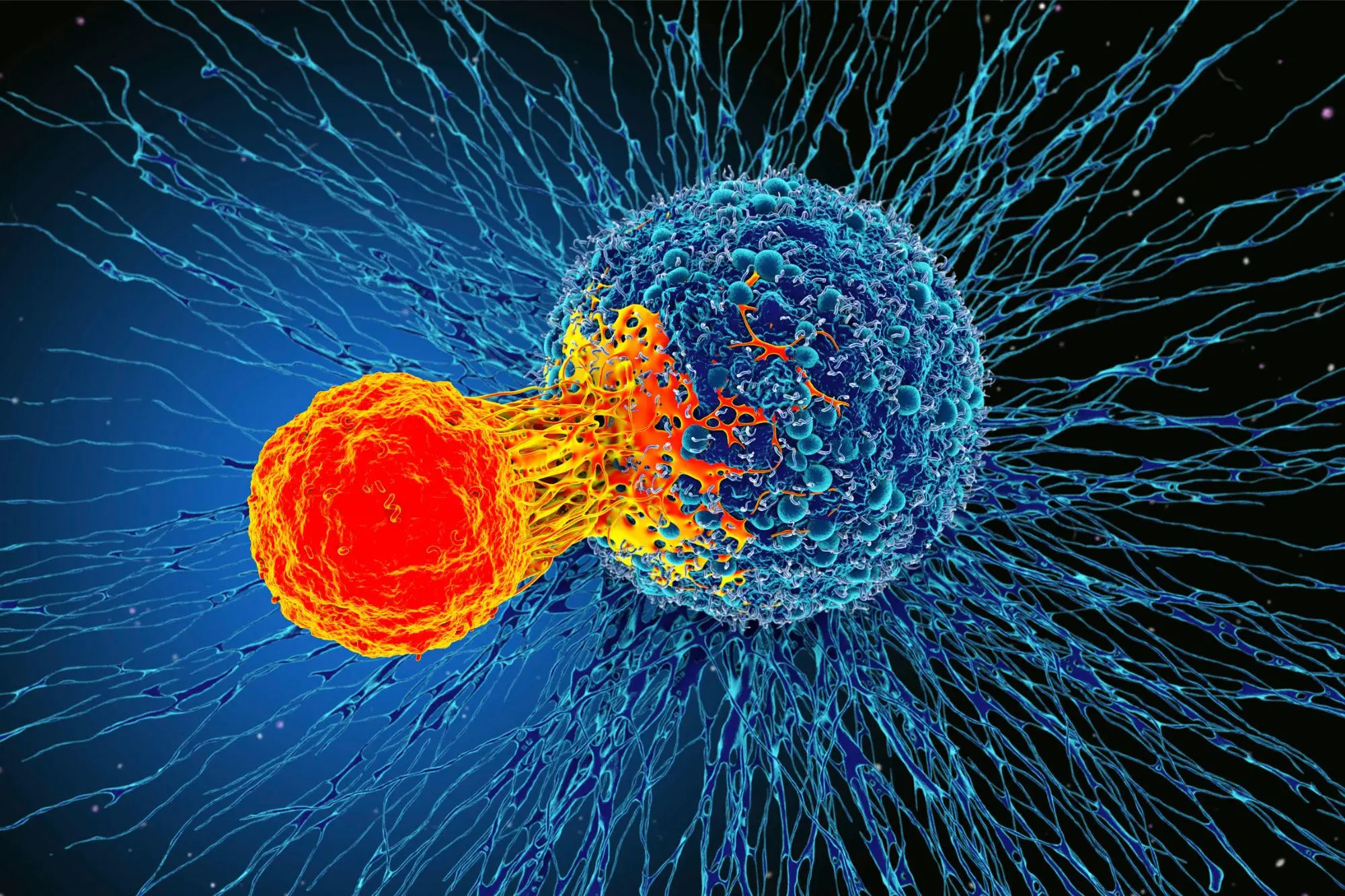
The study, published in Nature, analyzed immune responses in patients with metastatic melanoma receiving anti-PD-1 “checkpoint inhibitor” immunotherapy, using advanced gene-editing technology to make unprecedented observations about T-cell answers.
The study’s findings revealed that effective immunotherapy directs a diverse repertoire of T cells against a small group of selected mutations in a tumor. These T-cell responses expand and evolve during treatment within cancer and the bloodstream. Patients for whom the therapy fails also present a T-cell response against a similarly reduced number of mutations in cancer. Still, those immune responses are less focused and do not expand during treatment.
However, even in patients who don’t exhibit any visible clinical response to treatment, immune responses could be enhanced by isolating tumor-reactive T cells and modifying larger T cells to redirect them against the patient’s tumor. These T cells could be expanded in culture and reinfused into the patients to treat their tumors.
Improved Personalized Treatments:
“These findings mark an important step forward in understanding what the T-cell responses see in the tumor and how they change over time while they are in the tumor and circulation in the blood, searching for new tumor cells to attack,” said Cristina Puig-Saus, Ph.D., a UCLA Jonsson Comprehensive Cancer Center researcher and the first author of the study.
With a deeper understanding of how the T-cell responses clear metastatic tumor masses, scientists can engineer T cells in multiple ways to mimic them, potentially creating more effective, personalized immunotherapies.
Antoni Ribas, M.D., Ph.D., a UCLA Jonsson Comprehensive Cancer Center researcher and a co-senior author of the study, said that the study paves the way for scientists to know exactly what the immune system of a particular patient recognized in their cancer to differentiate it from normal cells and attack it.
“This study demonstrates that patients without response to therapy still induce a tumor-reactive T-cell response,” Puig-Saus said. “These T cells could be isolated, and their immune receptors used to genetically modify a larger number of T cells to redirect them against the patient’s tumor. These T cells could be expanded in culture and reinfused into the patients to treat their tumors.”
The study builds on technology developed through a collaboration between Ribas, James Heath, Ph.D., president of the Institute for Systems Biology in Seattle, and David Baltimore, Ph.D., Nobel laureate, emeritus professor at Caltech, and a member of the UCLA Jonsson Comprehensive Cancer Center.
The National Institutes of Health and PACT Pharma funded the study.


Comments are closed